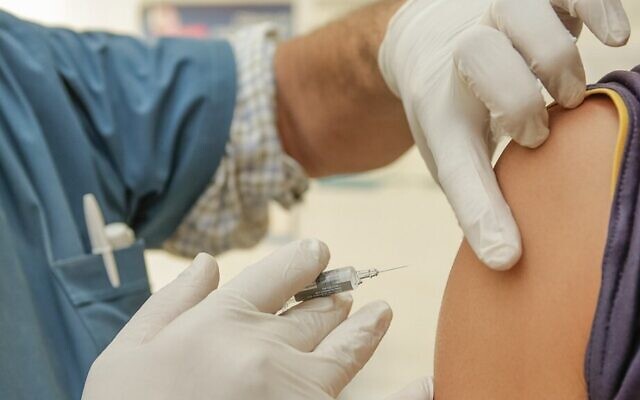
Two COVID-19 vaccines have been about 95% effective in preventing the virus from infecting individuals during the vaccines’ trials, their developers recently announced.
News came on Nov. 16 that Cambridge-based Moderna created a mRNA vaccine that was 94.5% effective during its phase three study. Moderna has been working on this vaccine since early January, according to the company.
Pfizer and BioNTech announced on Nov. 9 that their shared vaccine candidate was 90% effective in preventing COVID-19 in individuals that have not previously contracted the virus. On Wednesday, the company announced that the vaccine is now 95% effective.
BioNTech, a German biotechnology company, said it began the Project “Lightspeed” in January to develop a COVID-19 vaccine.
In mid-March, BioNTech announced its collaboration on the vaccine with the American pharmaceutical company Pfizer. The vaccine that has reported a 90% effectivity rate this November is called BNT162b2, according to Pfizer.
Melanie Berkmen, an associate professor in the Department of Chemistry and Biochemistry at Suffolk University, is optimistic about this vaccine.
“A 90% effective vaccine is phenomenal,” Berkman said. “It is really great. In order to pass federal regulation for use, it has to pass 50% effectiveness or more, so this is way up in our wildest dreams in effectiveness.”
The skepticism on the internet surrounding the process of the creation of vaccines during this year has been partially due to the length of time taken to create one. According to Pfizer, the companies began working on this vaccine 10 months ago. However, vaccine development often takes around 10-15 years, according to The College of Physicians of Philadelphia..
Despite many vaccines taking years to develop, Berkmen said this vaccine has not been rushed, and that she is hopeful for its future.
“Although this is one of the fastest production times for a potential vaccine, I believe there are a lot of oversight mechanisms to make sure that it is going to be safe and effective,” said Berkmen.
Assuming this vaccine gets approved by the FDA and is passed by the Federal Emergency Authorization Act, Berkmen would “absolutely suggest that people get vaccinated” if this vaccine becomes widely available.
Julianne Larsen, a nurse practitioner at the Center for Counseling, Health and Wellness at Suffolk, also has an optimistic outlook on this new vaccine.
“I do think that there are a lot of very smart and experienced people involved in the development of the vaccine. I also feel comforted that there will be a lot of external eyes on the vaccine, because it has been a very rapid process,” said Larsen.
If this vaccine is to become widely available, Larsen believes there would be a measurable emotional impact on healthcare workers who get vaccinated. Healthcare workers have been presented with great danger while taking care of those who contract COVID-19, and earlier this year, 5.9% of adults hospitalized were health care personnel, according to the CDC.
“Many healthcare workers have been adversely affected by the virus and the concern about bringing the virus home to others in their household,” Larsen said. “So I think it will make a big difference if healthcare workers feel that they are protected, or mostly protected from the virus.”
Both Larsen and Berkmen said that the 90% effective vaccine developed by Pfizer and BioNTech is one of the first to use messenger RNA (mRNA) technology.
This technology works by injecting mRNA into the patient. The injected mRNA codes for part of the spike protein. The spike protein is an antigen for the SARS-CoV-2 virus, according to The University of Cambridge. An antigen is a substance that the human body recognizes as foreign, and thus mounts to an immune response to it, according to The National Cancer Institute.
The spike protein is a protein on the viral surface used to bind to and enter the human cell. The injected mRNA enters the patient’s cells and is used to produce the spike protein. This new protein is recognized as foreign, eliciting an immune response and providing protection from the virus, also according to The University of Cambridge.
“In comparison [to other vaccines], the production of an mRNA vaccine is much more simple, and that’s why [this] technology is on the leading edge of vaccines,” said Berkmen. “There’s at least 100 different vaccines being attempted worldwide, and this one is one of the first ones to show effectiveness, because of this mRNA technology is much faster.”