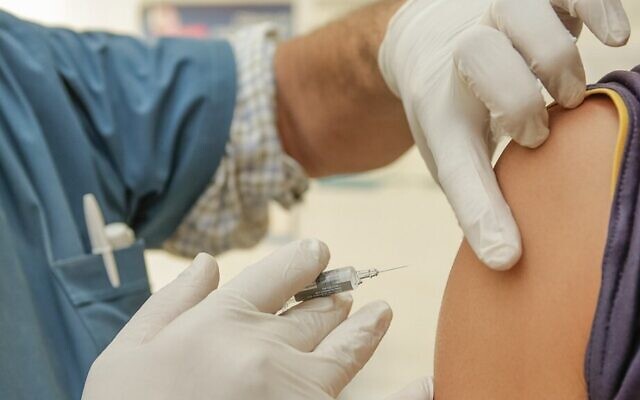
The CDC and FDA recommended a pause in the use of the Johnson & Johnson’s COVID-19 vaccine on April 13 after reports of a rare and severe blood clotting side effect.
Out of the nearly 7 million doses administered in the U.S., six recipients developed a rare blood clotting disorder within one to three weeks after vaccination, reported the New York Times. All the cases were reported in women ages 18 to 48.
“We are recommending a pause in the use of this vaccine out of an abundance of caution,” said Peter Marks, director of the Center for Biologics Evaluation and Research, in a joint statement from the CDC and FDA. “COVID-19 vaccine safety is a top priority for the federal government, and we take all reports of health problems following COVID-19 vaccination very seriously.”
The vaccine is still considered generally safe and will not cause severe side effects in most people, according to Johns Hopkins Bloomberg School of Public Health.
During this pause in distribution, the CDC and FDA will review the cases and data further, which could lead to updated vaccination policies and better clinical guidelines for the vaccine, according to Johns Hopkins.
Sarah Ross, a freshman psychology major at Suffolk University said that while the pause was necessary for further research, it was not necessarily an indication that the vaccine itself is unsafe.
“I honestly do not find it to be too worrisome, daily medication and things like birth control have way higher risk of blood clots and those are still taken everyday,” said Ross.
As of April 19, everyone over the age of 16 is eligible for the vaccine in Massachusetts.
Amelia Koval, a freshman environmental science major at the University of Vermont, received the Johnson & Johnson vaccine on April 12.
She received her shot unexpectedly during a trip to New Hampshire, and has not experienced any severe side effects thus far from the vaccine.
“When I found out about the pause, I freaked out a little,” said Koval. “I immediately called my pediatrician as well as my family members who are physicians.”
Koval explained that as a woman currently taking birth control, she was worried about the connection between birth control and blood clotting conditions. She decided to pause her birth control for two cycles.
“This is just a precaution that I am taking while more research is being done on the correlation between the vaccine and this clotting condition,” she said.
While the CDC is acting out of an abundance of caution, physicians are advising individuals that clotting caused from the vaccine versus birth control are different, reported NBC.
Despite her personal experience, Koval still believes that everyone should receive the vaccine if they are able.
“To me, getting vaccinated is synonymous with gaining back a sense of normalcy and a shot at enjoying a great summer without the same layer of anxiety that we have experienced for over a year,” Koval said.
While the side effect has only resulted in a small number of people, the CDC is advising individuals who received the vaccine within the last three weeks to monitor their symptoms and contact a medical professional if needed.
Follow Shea on Twitter!@ShealaghS