By Victoria Greenleaf
The Ebola virus continues to claim thousands of lives in Africa, but there is new hope arriving in the form of a new experimental vaccine made from the blood of former Ebola patients, according to BBC.
The World Health Organization announced the vaccine could begin testing on human subjects in West Africa by January, and a new blood serum could begin being used in Liberia in as little as two weeks, according to an article by The Guardian.
The final vaccine is being developed in a potential collaboration between Johnson & Johnson, a U.S. company, and GlaxoSmithKline, a U.K. company that has been working on a rivaling vaccine, according to an article published by Reuters.
Johnson & Johnson released a statement on Wednesday, Oct. 22, which outlined their hope to produce at least one million of their two-step vaccine plan by next year. Johnson & Johnson vaccine is to begin human trials in January, according to Reuters.
The WHO said it hoped to begin testing this vaccine, along with the other trial vaccines, on as many as 20,000 health workers and people in Liberia, Guinea, and Sierra Leone, the hot-zone countries of West Africa according to The Guardian.
BBC explained that if a person has successfully fought off Ebola, their body has learned how to combat the virus, and have developed certain antibodies that are immune. Scientists then use their blood and immune antibodies to craft the serum after removing the red blood cells, but keeping the antibodies intact.
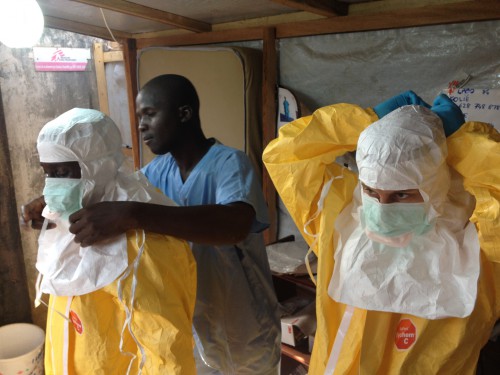
According to the Center for Disease Control, the Ebola virus has claimed more than 4,500 lives, but experts have predicted more than 10,000 cases a week could spring up in upcoming months if no new steps are taken to control the outbreak, according to BBC.
The CDC also explains that countries such as Spain and Senegal are at risk of travel-related infection. Both countries have already had one case each due to travel exposure, according to reports from BBC.
The U.S. has already had three infected persons, starting with Thomas Eric Duncan, a patient who had traveled from a hot-zone country to Dallas, Texas. Duncan died from the virus after apparently infecting two nurses caring for him, according to The Guardian.
In remarks reported to The Guardian, Dr. Marie Paule Kieny, assistant director general at WHO, said that there were many “ifs” remaining and that there is “still a possibility that it [a vaccine] will fail.”
“There are partnerships which are starting to be put in place to have capacity in the three countries to safely extract plasma and make preparation that can be used for the treatment of infective patients,” said Kieny.
“The partnership which is moving the quickest will be in Liberia where we hope that in the coming weeks there will be facilities set up to collect the blood, treat the blood and be able to process it for use,” said Dr. Kieny to BBC.
According to Reuters, although the vaccines’ safety is not proven, tests done on macaque monkeys infected with the Zaire, Ebola strain have proven to provide good protection, leading developers to believe that they will work as effectively on humans.